
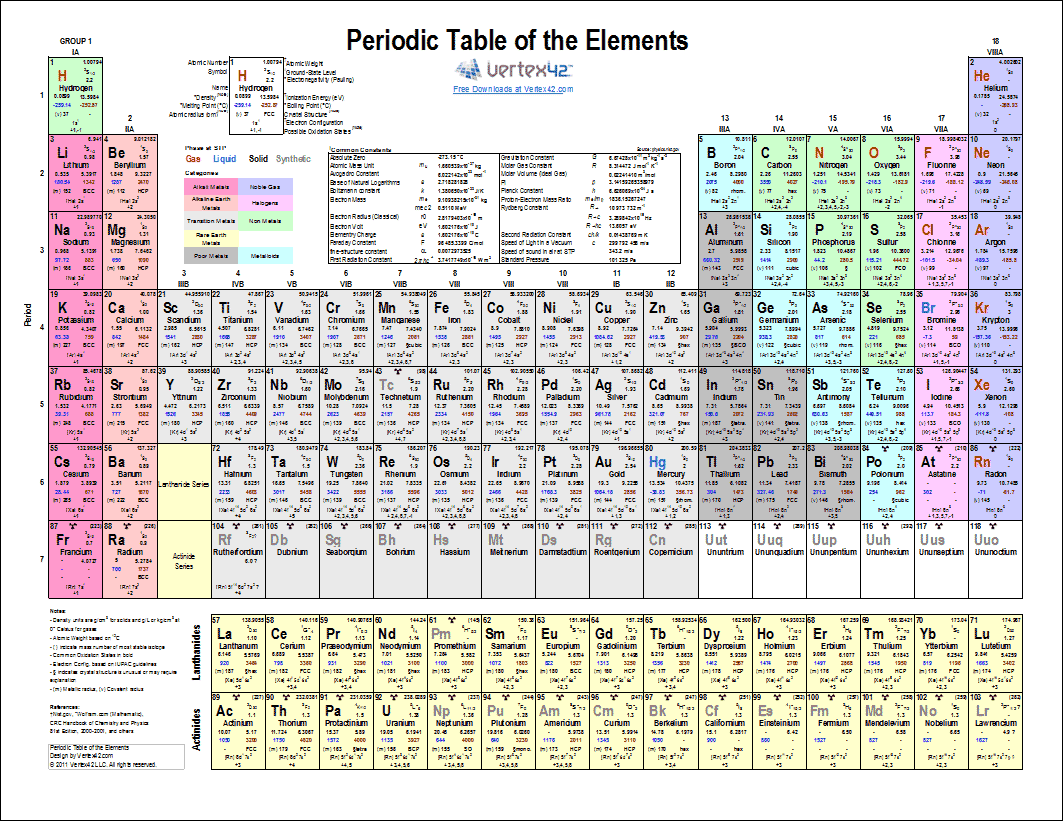

(l) the similarities and trends in physical and chemical properties of elements in the same group as illustrated by Group 1 and Group 7.(i) metals being found to the left and centre of the Periodic Table and non-metals to the right, with elements having intermediate properties appearing between the metals and non-metals in each period.(h) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.1.2 ATOMIC STRUCTURE AND THE PERIODIC TABLE.Unit 1: CHEMICAL SUBSTANCES, REACTIONS and ESSENTIAL RESOURCES.(k) the similarities and trends in physical and chemical properties of elements in the same group as illustrated by Group 1 and Group 7.(h) metals being found to the left and centre of the Periodic Table and non-metals to the right, with elements having intermediate properties appearing between the metals and non-metals in each period.(g) elements being arranged in order of increasing atomic number and in groups and periods in the modern Periodic Table, with elements having similar properties appearing in the same groups.

Ionization energy is the amount of energy required to remove an electron from an atom. All of these E’s increase going up and to the right of the periodic table.Įlectronegativity is the ability of an atom to attract electrons to itself at a covalent bond.Įlectron affinity is the energy associated when you add an electron to an atom. As you can see, basicity increases going up and to the left of the periodic table.Į stands for three things: Electronegativity, Electron affinity, and ionization Energy. B stands for basicity, the ability of molecules to accept protons. The mnemonic is bear: B E A R.Īnd each letter stands for different chemical properties. The periodic trends tell you in which direction of the periodic table do you have increasing values for different chemical properties. Today we have another general chemistry mnemonic for you and that mnemonic is for periodic trends. I’m Ken and I’m an MCAT expert with MedSchoolCoach. Welcome back to another MCAT Mnemonic Monday. Ken Tao is the MedSchoolCoach expert on MCAT, and uses the acronym “BEAR” to help you remember the periodic trends for basicity, electronegativity, electron affinity, and ionization energy, acidity and radius.Īll right.


 0 kommentar(er)
0 kommentar(er)
